+44 75754 30035 help@rapidassignmenthelp.co.uk
offer
🎁Special Offer 🎁 Discounts - Up to 55% OFF!
🎁Special Offer 🎁 Discounts - Up to 55% OFF!
This question explores a viral disease in the St Kilda Field mice, focusing on how it spreads within an isolated population. It covers infection dynamics, R₀ calculations, herd immunity, and the SI model for disease transmission
A basic reproduction number is the R₀, which measures how contagious an infectious disease is (Delamater et al., 2019). More specifically, it informs us how many other animals, on average, another animal inoculated with that infection will transfer the disease to within a population where everyone is not immune.
For instance, if R₀ is 2, that implies that one infected St Kilda Field mouse will continue to infect two more mice, and each of these two more will infect two more, resulting in exponential spread (Ramirez, 2020). Concepts like this are often used in assignments writing help to demonstrate calculations of disease transmission and herd immunity.
Herd immunisation protects a populace when a sizable piece is invulnerable because of inoculation or past contamination (Bullen, Heriot and Jamrozik, 2023). This principle is central in Veterinary and Zoonotic Infectious Diseases, as it helps prevent the disease from spreading swiftly, even among unvaccinated individuals. This helps prevent the disease from spreading swiftly, as relatively few hosts are susceptible to it. However, in this case, it is also suitable for unvaccinated individuals because the infection is more complicated to spread.
To prevent the spread of the disease, calculate the proportion of the population that needs to be vaccinated using the formula:
P(vac) = 1 – 1/R₀
Given:
R₀ = 2
P(vacc) = 1 – (1/2) = 0.5
The mouse population must be vaccinated so that 50 per cent can end the disease spreading.
The population of St Kilda Field mice is 1,500.
Number of mice to vaccinate is 0.5 × 1,500 = 750 mice.
The answer is 750 St Kilda Field mice would have to be vaccinated to be protected against the colony.
We use the SI model, which assumes that once infected, mice stay infected.
Formula: I(t+1) = I(t) + β × S(t) × I(t)
Where:
I(t) = number of infected at time t
S(t) = number of susceptible at time t
β = 0.1 (transmission rate per day
Starting values:
Day 0: I = 1, S = 1,499
The susceptible-infected (SI) model is one of the basic frameworks of epidemiology that models the pathology of the disease (Dhungana and Ghimire, 2021). Its (no other word will suffice) divides the population into two parts: (a) Susceptible (S), (b) Infected (I). This is a premise that makes it assumed that after infection individuals will continue to be infectious for the remainder of the timeline with no recovery or death.
Get assistance from our PROFESSIONAL ASSIGNMENT WRITERS to receive 100% assured AI-free and high-quality documents on time, ensuring an A+ grade in all subjects.
Strengths of the SI Model:
This model applies to isolated populations, such as St Kilda, with a defined mouse population and no immigration or emigration (Knopoff et al., 2022).
Limitations:
Implications for St Kilda vs Mainland: The model is more accurate on St Kilda because of a minor, isolated population, a known population size, and the birds' limited movement. Homogenous mixing may approximate reality rather well, provided that mice interact closely with each other (Foley et al., 2023).
The SI model would be less realistic on the mainland, which has large populations, complex habitats, and variable population densities. All of this complicates the picture. Transmission is not just movement between regions but between regions. Furthermore, there are varying contact rates, and sometimes predators can be present (Itescu et al., 2019). In such a context, models such as SIR (Susceptible-Infected-Recovered) or SEIR (additional exposed compartment) would provide better predictive accuracy.
This question examines the relationship between rodent populations and human GCD cases. It identifies which species act as reservoirs and analyzes how fluctuations in rodent abundance influence disease transmission.
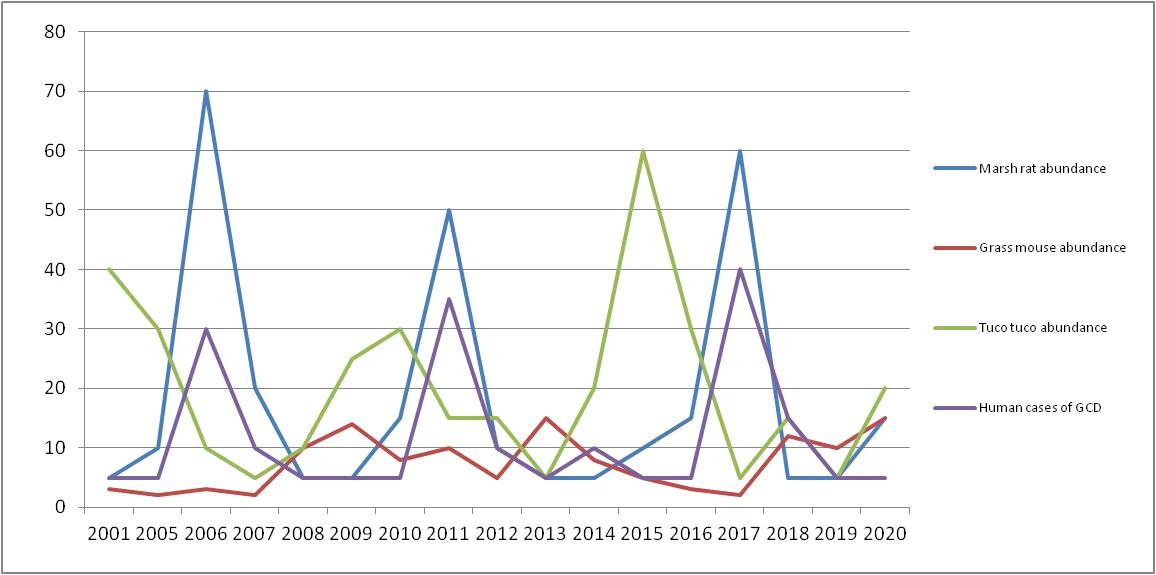
Figure 1: Rodent Abundance and Human GCD Cases (2001–2020)
This graph provides a visual of rodent abundance (per hectare) of Marsh Rats, Grass Mice, Tuco Tucos, and annual GCD cases from 2001–2020 reported by humans. There are clear trends showing that Marsh Rat abundance correlates most closely with the increase in spikes in human GCD cases, especially in 2006, 2011 and 2017.
The evidence clearly shows that Francisella brownie has a rodent reservoir and that the Marsh Rat (Holochilus chacarius) is the principal reservoir. This highlights a key topic in Veterinary and Zoonotic Infectious Diseases, demonstrating how animal hosts maintain pathogens that can spill over into humans. The conclusion is derived based on data from Tables 1 & 2 and Figure 1:
Correlation with Human Cases (Table 1 & Graph):
Human cases in the years of GCD closely follow peaks of high Marsh Rat abundance. There is no consistent correlation between Grass Mouse and Tuco Tuco with GCD cases.
Infection Prevalence (Table 2):
Across all years, the infection rates were significantly higher in Marsh Rats.
2020: 74%
2021: 87%
2022: 51%
2023: 60%
In contrast:
Grass Mice: 7–14%
Tuco Tucos: 4–19%
This, however, suggests the much greater responsibility of Marsh Rats in maintaining and disseminating the infection.
Genetic Analysis (Figure 1):
At the bacterial locus doss1, Marsh Rats contained all 11 genetic alleles. A total of six alleles were unique to Marsh Rats and exhibited genetic diversity and long-term association.
Three shared human alleles with Marsh Rats and two found in unrelated rodents suggest that Marsh Rats are likely an important source while other species fill the role of transport host.
Targeted Marsh Rat Control (Rodent Population Management):
Environmental Hygiene and Rodent-Proofing on Farms:
Public Health Surveillance and Education Campaigns:
This question investigates how projected climate changes affect malaria risk across different African regions. It highlights the influence of temperature, rainfall, and environmental changes on vector populations and disease spread.
The figure depicts projections of the number of human populations at risk of malaria in Central, Eastern, Southern and Western African regions under severe climate change scenarios for 2020, 2030, 2050 and 2080. Although the risk of malaria in Central Africa will remain low at 3.1 million by 2040, it will increase from 2.9 million in 2020 to 106 million in 2030 before declining to 5 million in 2080 (Kovats et al., 2018). Initial warming may give a competitive advantage to malaria transmission, but higher or different temperatures or ecological changes may also reduce mosquito suitability or lead to population displacement.
The population at risk in eastern Africa continues to increase steadily throughout the projection period. From around 30 million people in 2020, the risk population rises to nearly 100 million by 2080 (Kovats et al., 2018).
All decades have had a low and stable population at risk in southern Africa. The cause might include a persistent arid environment, vector lack or effective disease control measures.
The risk population has drastically declined from an estimated 100 million in 2020 down to less than 20 million in 2080 in western Africa (Kovats et al., 2018). In terms of future potential, this reversal implies that the area may become less friendly to malaria vectors as a result of environmental change or higher public health interventions.
Many vector-borne zoonotic diseases are affected by ecological dynamics and transmission potential, which are, in turn, caused by climate change. Rift Valley Fever is an example of a disease that affects humans and livestock and is mosquito-borne—primarily in Africa (Tinto et al., 2023). The disease vectors' habitats will expand, and their geographic locations will change as global temperatures rise and rainfall patterns become more erratic.
Temperature can affect mosquito development and the number of bites, both of which increase the likelihood of disease transmission. In addition, high temperatures can reduce the extrinsic incubation period of the virus within the mosquito so that the vector becomes more quickly infectious (Tinto et al., 2023). Mosquitoes can thrive in regions that used to be too cold or dry for mosquito survival, which could allow diseases like Rift Valley Fever to emerge elsewhere.
Surveillance in Human Populations
Given the challenges associated with disease surveillance in human populations, the main hurdle is restricted access to healthcare infrastructure, particularly in rural or underserved areas. Many affected communities may fail to detect, monitor, and respond to disease outbreaks due to the absence of diagnostic facilities, trained medical personnel, or reliable information reporting systems (Chemison et al., 2024).
Also, the under-reporting of diseases is due to social and cultural barriers. People may refrain from seeking medical care in some communities because of stigma, lack of trust or traditional beliefs about illness (Birungi et al., 2021). Due to this, surveillance data are limited in accuracy, and early intervention efforts are slowed down.
Surveillance in Wildlife Populations
In addition, wildlife disease surveillance is far more complex because its hosts are elusive and dispersed far and wide. Some species are difficult to monitor as they are nocturnal, migratory, and found in far away, inaccessible areas (Chemison et al., 2024). Such work needs to be carried out on the field, which is very logistically and resource-intensive.
References
Introduction This report is intended to offer financial strategy in terms of conducting financial projections, investment...View and Download
Introduction - Optimisation and Decision Modelling Assignment Answers The Optimisation and Decision Modelling Assignment deals...View and Download
Introduction: Sony Corporation’s Decision-Making Approach This paper investigates Sony Corporation’s decision-making...View and Download
Task 1 Role and responsibility in health and social care settings In health and social care setting, the role and...View and Download
Task 1 Achieve academic excellence with Rapid Assignment Help, offering expertly written and affordable Assignment Help. Answer...View and Download
Q.1. Advice to Lawrence regarding leave of the property For property lawyers, it is of paramount importance to be able to...View and Download
